The JOURNAL OF CRANIOMANDIBULAR PRACTICE June '84-Aug. 'b4, Vol. 2, No. 3
The Omohyoideus Myofascial Pain
Syndrome: Report of Four Patients
|

WELCOME..."LIFE...AND THE THINGS IT BRINGS" SOMETIMES, OUR LIVES CAN BE TURNED UPSIDE DOWN AND IT IS UP TO US & THE CHOICES, WE MAKE...THAT WILL DETERMINES THE OUTCOME. HOW WE HANDLE IT IS IMPORTANT, FOR THE CHOICES WE MAKE..DO MAKE A DIFFERENCE. LIFE,IS WHAT WE MAKE IT. SO,LET US ALL MAKE LIFE GOOD. ALWAYS "PAY IT FORWARD". WE CAN DO IT. :) DO TO OTHERS, AS YOU WOULD HAVE DONE TO YOU. GOD BLESS..
Saturday, October 15, 2011
MYOFASCIAL PAIN SYNDROME(OMOHYOIDEUS-4 patients reports)
Posted by
skeet65
at
3:37 PM
Thursday, October 6, 2011
Posted by
skeet65
at
11:01 AM
Your brain on religion
MRI white matter lesions
Many times I get consulted by patients or their relatives when their MRI brain report reads multiple scattered white matter lesions seen. The radiologist’s report usually further reads that these can be seen in primary demyelinating conditions like multiple sclerosis or in vascular disorders. Patient’s and caregivers are naturally worried when they get this MRI report and do not know what to do and how to proceed further. So I thought that here I shall talk about these white matter abnormalities seen on the MRI. What is their significance? Do they represent evidence of multiple sclerosis?
White matter signal changes on the MRI essentially means that on the MRI, the white matter showed some scattered bright spots. White matter in the brain refers to the fiber tracts that carry information to and fro from the brain.
My first question when somebody asks me what next and what does this mean is to ask them why was the MRI done in the first place. If the MRI was done because there was a clinical suspicion of multiple sclerosis then these white matter lesions may indeed have significance and may represent radiological evidence of MS plaques. Let me explain this with an example. You go to your doctor, you have signs and symptoms that suggest MS (example you may have had an attack of optic neuritis), when the doctor examines you he is able to elict signs in the examination compatible with a diagnosis of MS, then he orders an MRI to see if you do have evidence of white matter lesions in the brain. In a case like this the presence of white matter lesions/ signal changes in the MRI is obviously important. Here it likely does suggest the presence of MS. That said and done I again want to re-emphasize that the diagnosis of MS is made on the basis of clinical history of previous attacks, CSF (spinal fluid) examination and MRI, not just on the basis of the MRI alone. Also there are certain criteria which have to be satisfied on MRI to make a definite diagnosis of MS. These radiological criteria for MS include the number of lesions on the MRI, their location and their size.
Thus it is important to remember that a person who is noted to have white matter lesions on a brain MRI does not necessarily have MS. White matter lesions can be seen in numerous other conditions and they are more commonly seen as we grow older. The thinking behind this is that they represent microvascular ischemic changes in the brain (the smaller caliber blood vessels in the brain showing signs of ischemia or decreased blood flow). Hence these white matter abnormalities on MRI are more commonly seen in patients who have microvascular and macrovascular risk factors such as a history of hypertension, diabetes and high cholesterol (dyslipidemia/ bad lipid profile).
White matter signal changes on MRI may also be seen in patients who have infectious and other inflammatory conditions. They have been reported in the MRI of patients with a history of migraine headaches (migraine too is a vascular disorder and that may explain the connection).
So I want to end by saying that the presence of these white matter signal changes on brain MRI has to be correlated to the history, clinical examination and other ancillary investigations. Your doctor shall help you in going about this in a methodical manner. I repeat these white matter lesions do not suggest MS in each and every case they are found.
White matter signal changes on the MRI essentially means that on the MRI, the white matter showed some scattered bright spots. White matter in the brain refers to the fiber tracts that carry information to and fro from the brain.
My first question when somebody asks me what next and what does this mean is to ask them why was the MRI done in the first place. If the MRI was done because there was a clinical suspicion of multiple sclerosis then these white matter lesions may indeed have significance and may represent radiological evidence of MS plaques. Let me explain this with an example. You go to your doctor, you have signs and symptoms that suggest MS (example you may have had an attack of optic neuritis), when the doctor examines you he is able to elict signs in the examination compatible with a diagnosis of MS, then he orders an MRI to see if you do have evidence of white matter lesions in the brain. In a case like this the presence of white matter lesions/ signal changes in the MRI is obviously important. Here it likely does suggest the presence of MS. That said and done I again want to re-emphasize that the diagnosis of MS is made on the basis of clinical history of previous attacks, CSF (spinal fluid) examination and MRI, not just on the basis of the MRI alone. Also there are certain criteria which have to be satisfied on MRI to make a definite diagnosis of MS. These radiological criteria for MS include the number of lesions on the MRI, their location and their size.
Thus it is important to remember that a person who is noted to have white matter lesions on a brain MRI does not necessarily have MS. White matter lesions can be seen in numerous other conditions and they are more commonly seen as we grow older. The thinking behind this is that they represent microvascular ischemic changes in the brain (the smaller caliber blood vessels in the brain showing signs of ischemia or decreased blood flow). Hence these white matter abnormalities on MRI are more commonly seen in patients who have microvascular and macrovascular risk factors such as a history of hypertension, diabetes and high cholesterol (dyslipidemia/ bad lipid profile).
White matter signal changes on MRI may also be seen in patients who have infectious and other inflammatory conditions. They have been reported in the MRI of patients with a history of migraine headaches (migraine too is a vascular disorder and that may explain the connection).
So I want to end by saying that the presence of these white matter signal changes on brain MRI has to be correlated to the history, clinical examination and other ancillary investigations. Your doctor shall help you in going about this in a methodical manner. I repeat these white matter lesions do not suggest MS in each and every case they are found.
Dr. Sethi
Visit and participitate in my blog:
http://braindiseases.wordpress.com
Visit the brain care foundation website at:
www.braincarefoundation.com
THIS IS VERY INTERESTING, SINCE I DO HAVE WHITE MATTER ALL IN MY MRI BRAIN SCAN. IT IS A TERRIBLE PLACE TO BE, WHEN NOT TAKEN SERIOUSLY AND A PERSON HAS TO FIGHT TO GET AN ANSWER TO WHAT IS GOING ON.
I RECENTLY WENT TO A NEUROLOGIST THAT DIDN'T TAKE ME SERIOUSLY, FOR APPARENTLY WAS MOCKING ME AT MY LAST VISIT. IF IT ISN'T HARD ENOUGH TO MAKE IT THERE AND STAY IN THE LOBBY FOR 2 HOURS, THEN ANOTHER HOUR IN HIS ROOM, THEN WHEN HE FINALLY DOES COME IN, HE IS RUSHING AND GETTING THROUGH WITH THE OFFICE VISIT SO THAT HE CAN RUSH OVER TO ANOTHER ROOM. I KNOW, I HAVE BEEN THERE AND I AM TIRED OF THESE SO CALLED DOCTORS...WHO, ARE SUPPOSE TO HAVE OUR BEST INTEREST AT HEART. INSTEAD, THEY RIDICULE YOU AND SEND YOU OUT OF THEIR OFFICE WITH MORE QUESTIONS THAN, ANSWERS.
I JUST WANTED TO POST MY BRAIN MRI HERE, SO THAT WHEN I DO GET DIAGNOSED, I WILL HAVE EVIDENCE THAT THIS DOCTOR FOUND, WHAT HE SAW ON MY MRI, AS INSIGNIFICANT. IT IS WRONG AND BAD ENOUGH FOR A DOCTOR TO SEEM NOT TO CARE, BUT WHEN THEY ACTUALLY MAKE COMMENTS THAT ARE CONDESCENDING AND OFFENDING, THEN SOMETHING HAS TO BE DONE. THIS DOCTOR, THE LAST TIME, I WAS THERE, TOLD ME THIS, "YOU DON'T HAVE LEPROSY DO YOU?" AND "DO YOU HAVE A PROSTATE PROBLEM, DO YOU?" I TOLD HIM, AFTER HE SAID THAT, THAT "I DON'T KNOW, HAVE YOU CHECKED?"..HE SAID, "WOMEN DON'T HAVE PROSTATES.". THEN, I REPLIED, "I DO KNOW THAT!"
THIS OFFICE VISIT WAS UNREAL. I HAVE NEVER HEARD ANY DOCTOR TALK TO ME, THE WAY THAT HE DID. HE WAS VERY CONDESCENDING AND IT WAS VERY OFFESSIVE AND HURTFUL, TO ME, SINCE I DEAL WITH A GREAT AMOUNT OF PAIN. I STRUGGLE TO GET THROUGH EVERYDAY, AS IT IS AND I DON'T NEED SOMEONE WHO IS SUPPOSED TO BE PROFESSIONAL TREATS YOU LIKE YOU DON'T MATTER.
HE WAS TELLING ME THAT I HAD TOO MANY SYMPTOMS AND I TOLD HIM THAT I KNEW THAT I HAD A LOT AND ALL OF THE SYMPTOMS, AFTER A WHILE WILL MAKE ANY PERSON DEPRESSED. IF I DIDN'T HAVE ALL OF THIS PAIN AND SUFFERING AS I DO, EACH AND EVERYDAY...I WOULD NOT BE DEPRESSED, AT ALL. I WOULD BE TO BUSY LIVING MY LIFE, TO BE THAT WAY.
THE LAST TIME I WAS THERE HE GAVE ME A PRESCRIPTION FOR CYMBALTA, BUT I DID TAKE ONE AND MY SYMPTOMS INCREASED. IT SAID ON THE INSTRUCTIONS NOT TO CONTINUE, IF SYMPTOMS WORSENED. I TOLD HIM THAT. HE ACTUALLY GAVE ME ANOTHER PRESCRIPTION TO TRY AND I TOLD HIM THAT I DIDN'T WANT IT AND HE ASKED ME WHAT IT WAS THAT I WANTED. I TOLD HIM THAT ALL I WANTED WAS TO FIND OUT WHAT IT IS THAT FEELS LIKE IT'S KILLING ME.
SOMETHING HAS TO BE DONE AND THE ANSWERS HAVE TO BE FOUND. IF, WE...THE PATIENTS, DON'T PUSH TO FIND THE ANSWERS, TO WHAT IT IS THAT IS PLAGUING US, THEN WE ARE VERY POSSIBLY TAKING THE CHANCE ON BEING A STATISTIC. I DON'T KNOW @ YOU, BUT I DON'T WANT TO BE ONE...I JUST WANT MY LIFE BACK.
I AM SO TIRED, OF HOW SOME PEOPLE TREAT THOSE WHO ARE SUFFERING, JUST BECAUSE THEY DON'T UNDERSTAND OR WANT TO UNDERSTAND, DOESN'T GIVE THEM THE RIGHT TO PUNISH THE PERSON ANY FURTHER. WHY DO THEY RIDICULE THE PERSON WHO IS ALREADY HAVING SUCH A HARD TIME TRYING TO LIVE THEIR LIFE? LIFE IS HARD ENOUGH, BESIDES HAVING TO DEAL WITH AN "ASS"..(sorry, it is sad, but true)
I AM PRETTY SURE THAT THE MRI'S THAT I HAVE POSTED SHOW EVIDENCE ENOUGH OF WHITE MATTER. DO DOCTORS EVEN LOOK AT THESE THINGS???? I DO HAVE MORE THAT SHOW EVEN MORE THAT I FOUND AFTER I POSTED THESE, SO I DON'T UNDERSTAND WHY IS IT SO HARD TO EVEN GET INTO SEE A DOCTOR THAT CAN HELP YOU. I HAVE BEEN TRYING AND IT IS LIKE PULLING TEETH...NO, WORSE.
GOD BLESS EVERYONE AND I PRAY THAT IF THERE IS ANYONE ELSE LOOKING FOR ANSWERS, DON'T GIVE UP. WE HAVE TO FIGHT TO STAY ALIVE THESE DAYS.:)))<3 Karen
http://braindiseases.wordpress.com
Visit the brain care foundation website at:
www.braincarefoundation.com
THIS IS VERY INTERESTING, SINCE I DO HAVE WHITE MATTER ALL IN MY MRI BRAIN SCAN. IT IS A TERRIBLE PLACE TO BE, WHEN NOT TAKEN SERIOUSLY AND A PERSON HAS TO FIGHT TO GET AN ANSWER TO WHAT IS GOING ON.
I RECENTLY WENT TO A NEUROLOGIST THAT DIDN'T TAKE ME SERIOUSLY, FOR APPARENTLY WAS MOCKING ME AT MY LAST VISIT. IF IT ISN'T HARD ENOUGH TO MAKE IT THERE AND STAY IN THE LOBBY FOR 2 HOURS, THEN ANOTHER HOUR IN HIS ROOM, THEN WHEN HE FINALLY DOES COME IN, HE IS RUSHING AND GETTING THROUGH WITH THE OFFICE VISIT SO THAT HE CAN RUSH OVER TO ANOTHER ROOM. I KNOW, I HAVE BEEN THERE AND I AM TIRED OF THESE SO CALLED DOCTORS...WHO, ARE SUPPOSE TO HAVE OUR BEST INTEREST AT HEART. INSTEAD, THEY RIDICULE YOU AND SEND YOU OUT OF THEIR OFFICE WITH MORE QUESTIONS THAN, ANSWERS.
I JUST WANTED TO POST MY BRAIN MRI HERE, SO THAT WHEN I DO GET DIAGNOSED, I WILL HAVE EVIDENCE THAT THIS DOCTOR FOUND, WHAT HE SAW ON MY MRI, AS INSIGNIFICANT. IT IS WRONG AND BAD ENOUGH FOR A DOCTOR TO SEEM NOT TO CARE, BUT WHEN THEY ACTUALLY MAKE COMMENTS THAT ARE CONDESCENDING AND OFFENDING, THEN SOMETHING HAS TO BE DONE. THIS DOCTOR, THE LAST TIME, I WAS THERE, TOLD ME THIS, "YOU DON'T HAVE LEPROSY DO YOU?" AND "DO YOU HAVE A PROSTATE PROBLEM, DO YOU?" I TOLD HIM, AFTER HE SAID THAT, THAT "I DON'T KNOW, HAVE YOU CHECKED?"..HE SAID, "WOMEN DON'T HAVE PROSTATES.". THEN, I REPLIED, "I DO KNOW THAT!"
THIS OFFICE VISIT WAS UNREAL. I HAVE NEVER HEARD ANY DOCTOR TALK TO ME, THE WAY THAT HE DID. HE WAS VERY CONDESCENDING AND IT WAS VERY OFFESSIVE AND HURTFUL, TO ME, SINCE I DEAL WITH A GREAT AMOUNT OF PAIN. I STRUGGLE TO GET THROUGH EVERYDAY, AS IT IS AND I DON'T NEED SOMEONE WHO IS SUPPOSED TO BE PROFESSIONAL TREATS YOU LIKE YOU DON'T MATTER.
HE WAS TELLING ME THAT I HAD TOO MANY SYMPTOMS AND I TOLD HIM THAT I KNEW THAT I HAD A LOT AND ALL OF THE SYMPTOMS, AFTER A WHILE WILL MAKE ANY PERSON DEPRESSED. IF I DIDN'T HAVE ALL OF THIS PAIN AND SUFFERING AS I DO, EACH AND EVERYDAY...I WOULD NOT BE DEPRESSED, AT ALL. I WOULD BE TO BUSY LIVING MY LIFE, TO BE THAT WAY.
THE LAST TIME I WAS THERE HE GAVE ME A PRESCRIPTION FOR CYMBALTA, BUT I DID TAKE ONE AND MY SYMPTOMS INCREASED. IT SAID ON THE INSTRUCTIONS NOT TO CONTINUE, IF SYMPTOMS WORSENED. I TOLD HIM THAT. HE ACTUALLY GAVE ME ANOTHER PRESCRIPTION TO TRY AND I TOLD HIM THAT I DIDN'T WANT IT AND HE ASKED ME WHAT IT WAS THAT I WANTED. I TOLD HIM THAT ALL I WANTED WAS TO FIND OUT WHAT IT IS THAT FEELS LIKE IT'S KILLING ME.
SOMETHING HAS TO BE DONE AND THE ANSWERS HAVE TO BE FOUND. IF, WE...THE PATIENTS, DON'T PUSH TO FIND THE ANSWERS, TO WHAT IT IS THAT IS PLAGUING US, THEN WE ARE VERY POSSIBLY TAKING THE CHANCE ON BEING A STATISTIC. I DON'T KNOW @ YOU, BUT I DON'T WANT TO BE ONE...I JUST WANT MY LIFE BACK.
I AM SO TIRED, OF HOW SOME PEOPLE TREAT THOSE WHO ARE SUFFERING, JUST BECAUSE THEY DON'T UNDERSTAND OR WANT TO UNDERSTAND, DOESN'T GIVE THEM THE RIGHT TO PUNISH THE PERSON ANY FURTHER. WHY DO THEY RIDICULE THE PERSON WHO IS ALREADY HAVING SUCH A HARD TIME TRYING TO LIVE THEIR LIFE? LIFE IS HARD ENOUGH, BESIDES HAVING TO DEAL WITH AN "ASS"..(sorry, it is sad, but true)
I AM PRETTY SURE THAT THE MRI'S THAT I HAVE POSTED SHOW EVIDENCE ENOUGH OF WHITE MATTER. DO DOCTORS EVEN LOOK AT THESE THINGS???? I DO HAVE MORE THAT SHOW EVEN MORE THAT I FOUND AFTER I POSTED THESE, SO I DON'T UNDERSTAND WHY IS IT SO HARD TO EVEN GET INTO SEE A DOCTOR THAT CAN HELP YOU. I HAVE BEEN TRYING AND IT IS LIKE PULLING TEETH...NO, WORSE.
GOD BLESS EVERYONE AND I PRAY THAT IF THERE IS ANYONE ELSE LOOKING FOR ANSWERS, DON'T GIVE UP. WE HAVE TO FIGHT TO STAY ALIVE THESE DAYS.:)))<3 Karen
Sunday, October 2, 2011
Lumbar Puncture information just in case I do have Idiopathic Intracranial Hypertension
Posted by
skeet65
at
2:35 AM
The Wikimedia Foundation's 2nd steward election in 2011 has started. Please vote.
Lumbar puncture
From Wikipedia, the free encyclopedia
(Redirected from Lumber puncture)
Jump to: navigation, search
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. (December 2007) |
| Lumbar puncture | |
|---|---|
| Intervention | |
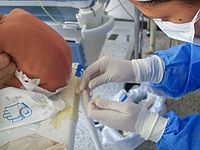
 A patient undergoes a lumbar puncture at the hands of a neurologist. The reddish-brown swirls on the patient's back are tincture of iodine (an antiseptic). | |
| ICD-9-CM | 03.31 |
| MeSH | D013129 |
Contents[hide] |
[edit] Indications
The most common purpose for a lumbar puncture is to collect cerebrospinal fluid in a case of suspected meningitis, since there is no other reliable tool with which meningitis, a life-threatening but highly treatable condition, can be excluded. Young infants commonly require lumbar puncture as a part of the routine workup for fever without a source, as they have a much higher risk of meningitis than older persons and do not reliably show signs of meningeal irritation (meningismus). In any age group, subarachnoid hemorrhage, hydrocephalus, benign intracranial hypertension and many other diagnoses may be supported or excluded with this test.Lumbar punctures may also be done to inject medications into the cerebrospinal fluid ("intrathecally"), particularly for spinal anesthesia or chemotherapy. It may also be used to detect the presence of malignant cells in the CSF, as in carcinomatous meningitis or medulloblastoma.
[edit] Contraindications
Lumbar puncture should not be performed in the following situations- Idiopathic (unidentified cause) increased intracranial pressure (ICP)
- Rationale: lumbar puncture in the presence of increased ICP may cause uncal herniation
- Exception: therapeutic use of lumbar puncture to reduce ICP
- Precaution
- CT brain is advocated by some, especially in the following situations
- Age >65
- Reduced GCS or conscious state
- Recent history of seizure
- Focal neurological signs
- Ophthalmoscopy for papilledema
- CT brain is advocated by some, especially in the following situations
- Bleeding diathesis
- Coagulopathy
- Decreased platelet count (<50 x 109/L)
- Infections[1]
- Skin infection at puncture site
- Sepsis
- Abnormal respiratory pattern
- Hypertension with bradycardia and deteriorating consciousness
- Vertebral deformities (scoliosis or kyphosis), in hands of an inexperienced physician.[2][3]
[edit] Procedure
In performing a lumbar puncture, first the patient is usually placed in a left (or right) lateral position with his/her neck bent in full flexion and knees bent in full flexion up to his/her chest, approximating a fetal position as much as possible. It is also possible to have the patient sit on a stool and bend his/her head and shoulders forward. The area around the lower back is prepared using aseptic technique. Once the appropriate location is palpated, local anaesthetic is infiltrated under the skin and then injected along the intended path of the spinal needle. A spinal needle is inserted between the lumbar vertebrae L3/L4 or L4/L5 and pushed in until there is a "give" that indicates the needle is past the ligamentum flavum. The needle is again pushed until there is a second 'give' that indicates the needle is now past the dura mater. Since the arachnoid membrane and the dura mater exist in flush contact with one another in the living person's spine (due to fluid pressure from CSF in the subarachnoid space pushing the arachnoid membrane out towards the dura), once the needle has pierced the dura mater it has also traversed the thinner arachnoid membrane and is now in the subarachnoid space. The stylet from the spinal needle is then withdrawn and drops of cerebrospinal fluid are collected. The opening pressure of the cerebrospinal fluid may be taken during this collection by using a simple column manometer. The procedure is ended by withdrawing the needle while placing pressure on the puncture site. In the past, the patient would often be asked to lie on his/her back for at least six hours and be monitored for signs of neurological problems, though there is no scientific evidence that this provides any benefit. The technique described is almost identical to that used in spinal anesthesia, except that spinal anesthesia is more often done with the patient in a seated position.The upright seated position is advantageous in that there is less distortion of spinal anatomy which allows for easier withdrawal of fluid. It is preferred by some practitioners when a lumbar puncture is performed on an obese patient where having them lie on their side would cause a scoliosis and unreliable anatomical landmarks. On the other hand, opening pressures are notoriously unreliable when measured on a seated patient and therefore the left or right lateral (lying down) position is preferred if an opening pressure needs to be measured.
Patient anxiety during the procedure can lead to increased CSF pressure, especially if the person holds their breath, tenses their muscles or flexes their knees too tightly against their chest. Diagnostic analysis of changes in fluid pressure during lumbar puncture procedures requires attention both to the patient's condition during the procedure and to their medical history.[citation needed]
Reinsertion of the stylet may decrease the rate of post lumbar puncture headaches.[3]
[edit] Risks
Post spinal headache with nausea is the most common complication; it often responds to analgesics and infusion of fluids. It was long taught that this complication can often be prevented by strict maintenance of a supine posture for two hours after the successful puncture; this has not been borne out in modern studies involving large numbers of patients. Merritt's Neurology (10th edition), in the section on lumbar puncture, notes that intravenous caffeine injection is often quite effective in aborting these so-called "spinal headaches." Contact between the side of the lumbar puncture needle and a spinal nerve root can result in anomalous sensations (paresthesia) in a leg during the procedure; this is harmless and patients can be warned about it in advance to minimize their anxiety if it should occur. A headache that is persistent despite a long period of bedrest and occurs only when sitting up may be indicative of a CSF leak from the lumbar puncture site. It can be treated by more bedrest, or by an epidural blood patch, where the patient's own blood is injected back into the site of leakage to cause a clot to form and seal off the leak.Serious complications of a properly performed lumbar puncture are extremely rare.[citation needed] They include spinal or epidural bleeding, and trauma to the spinal cord or spinal nerve roots resulting in weakness or loss of sensation, or even paraplegia. The latter is exceedingly rare, since the level at which the spinal cord ends (normally the inferior border of L1, although it is slightly lower in infants) is several vertebral spaces above the proper location for a lumbar puncture (L3/L4). There are case reports of lumbar puncture resulting in perforation of abnormal dural arterio-venous malformations, resulting in catastrophic epidural hemorrhage; this is exceedingly rare.
The procedure is not recommended when epidural infection is present or suspected, when topical infections or dermatological conditions pose a risk of infection at the puncture site or in patients with severe psychosis or neurosis with back pain. Some authorities believe that withdrawal of fluid when initial pressures are abnormal could result in spinal cord compression or cerebral herniation; others believe that such events are merely coincidental in time, occurring independently as a result of the same pathology that the lumbar puncture was performed to diagnose. In any case, computed tomography of the brain is often performed prior to lumbar puncture if an intracranial mass is suspected.
Removal of cerebrospinal fluid resulting in reduced fluid pressure has been shown to correlate with greater reduction of cerebral blood flow among patients with Alzheimer's disease. Its clinical significance is uncertain.
[edit] Diagnostics
Increased CSF pressure can indicate congestive heart failure, cerebral edema, subarachnoid hemorrhage, hypo-osmolality resulting from hemodialysis, meningeal inflammation, purulent meningitis or tuberculous meningitis, hydrocephalus, or pseudotumor cerebri.Decreased CSF pressure can indicate complete subarachnoid blockage, leakage of spinal fluid, severe dehydration, hyperosmolality, or circulatory collapse. Significant changes in pressure during the procedure can indicate tumors or spinal blockage resulting in a large pool of CSF, or hydrocephalus associated with large volumes of CSF. Lumbar puncture for the purpose of reducing pressure is performed in some patients with idiopathic intracranial hypertension (also called pseudotumor cerebri.)
The presence of white blood cells in cerebrospinal fluid is called pleocytosis. A small number of monocytes can be normal; the presence of granulocytes is always an abnormal finding. A large number of granulocytes often heralds bacterial meningitis. White cells can also indicate reaction to repeated lumbar punctures, reactions to prior injections of medicines or dyes, central nervous system hemorrhage, leukemia, recent epileptic seizure, or a metastatic tumor. When peripheral blood contaminates the withdrawn CSF, a common procedural complication, white blood cells will be present along with erythrocytes, and their ratio will be the same as that in the peripheral blood.
The finding of erythrophagocytosis,[4] where phagocytosed erythrocytes is observed, signifies haemorrhage into the CSF that preceded the lumbar puncture. Therefore, when erythrocytes are detected in the CSF sample, erythrophagocytosis suggests causes other than a traumatic tap, such as intracranial haemorrhage and haemorrhagic herpetic encephalitis. In which case, further investigations are warranted, including imaging and viral culture.
Several substances found in cerebrospinal fluid are available for diagnostic measurement.
- Measurement of chloride levels may aid in detecting the presence of tuberculous meningitis.
- Glucose is usually present in the CSF; the level is usually about 60% that in the peripheral circulation. A fingerstick or venipuncture at the time of lumbar puncture may therefore be performed to assess peripheral glucose levels in order to determine a predicted CSF glucose value. Decreased glucose levels can indicate fungal, tuberculous or pyogenic infections; lymphomas; leukemia spreading to the meninges; meningoencephalitic mumps; or hypoglycemia. A glucose level of less than one third of blood glucose levels in association with low CSF lactate levels is typical in hereditary CSF glucose transporter deficiency also known as De Vivo disease.
- Increased glucose levels in the fluid can indicate diabetes, although the 60% rule still applies.
- Increased levels of glutamine are often involved with hepatic encephalopathies, Reye's syndrome, hepatic coma, cirrhosis and hypercapnia.
- Increased levels of lactate can occur the presence of cancer of the CNS, multiple sclerosis, heritable mitochondrial disease, low blood pressure, low serum phosphorus, respiratory alkalosis, idiopathic seizures, traumatic brain injury, cerebral ischemia, brain abscess, hydrocephalus, hypocapnia or bacterial meningitis.
- The enzyme lactate dehydrogenase can be measured to help distinguish meningitides of bacterial origin, which are often associated with high levels of the enzyme, from those of viral origin in which the enzyme is low or absent.
- Changes in total protein content of cerebrospinal fluid can result from pathologically increased permeability of the blood-cerebrospinal fluid barrier, obstructions of CSF circulation, meningitis, neurosyphilis, brain abscesses, subarachnoid hemorrhage, polio, collagen disease or Guillain-Barré syndrome, leakage of CSF, increases in intracranial pressure or hyperthyroidism. Very high levels of protein may indicate tuberculous meningitis or spinal block.
- IgG synthetic rate is calculated from measured IgG and total protein levels; it is elevated in immune disorders such as multiple sclerosis, transverse myelitis, and neuromyelitis optica of Devic.
- Numerous antibody-mediated tests for CSF are available in some countries: these include rapid tests for antigens of common bacterial pathogens, treponemal titers for the diagnosis of neurosyphilis and Lyme disease, Coccidioides antibody, and others.
- The India ink test is still used for detection of meningitis caused by Cryptococcus neoformans, but the cryptococcal antigen (CrAg) test has a higher sensitivity.
- CSF can be sent to the microbiology lab for various types of smears and cultures to diagnose infections.
- Polymerase chain reaction (PCR) has been a great advance in the diagnosis of some types of meningitis. It has high sensitivity and specificity for many infections of the CNS, is fast, and can be done with small volumes of CSF. Even though testing is expensive, it saves cost of hospitalization.
[edit] History
The first technique for accessing the dural space was described by the London physician Dr Walter Essex Wynter. In 1889, he developed a crude cut down with cannulation in 4 patients with tuberculous meningitis. The main purpose was the treatment of raised intracranial pressure rather than for diagnosis.[5] The technique for needle lumbar puncture was then introduced by the German physician Heinrich Quincke, who credits Wynter with the earlier discovery; he first reported his experiences at an internal medicine conference in Wiesbaden in 1891.[6] He subsequently published a book on the subject.[7][8]The lumbar puncture procedure was taken to the United States by Arthur H. Wentworth M.D., an assistant professor at the Harvard Medical School, based at Children's Hospital. In 1893, he published a long paper on diagnosing cerebro-spinal meningitis by examining spinal fluid. His career took a nosedive, however, when antivivisectionists prosecuted him for having obtained spinal fluid from children. He was acquitted, but he was disinvited from the then forming Johns Hopkins Medical School where he would have been the first professor of pediatrics.[citation needed]
[edit] References
- ^ Mary Louise Turgeon (2005). Clinical hematology: theory and procedures. Lippincott Williams & Wilkins. pp. 401–. ISBN 9780781750073. http://books.google.com/books?id=cHAjsUgegpQC&pg=PA401. Retrieved 28 October 2010.
- ^ Roos KL (March 2003). "Lumbar puncture". Semin Neurol 23 (1): 105–14. doi:10.1055/s-2003-40758. PMID 12870112.
- ^ a b Straus SE, Thorpe KE, Holroyd-Leduc J (October 2006). "How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis?". JAMA 296 (16): 2012–22. doi:10.1001/jama.296.16.2012. PMID 17062865.
- ^ Harald Kluge (2007). Atlas of CSF cytology. Thieme. pp. 45–46. ISBN 9783131431615. http://books.google.com/books?id=HDLv-LAfqHoC&pg=PA45. Retrieved 28 October 2010.
- ^ Wynter WE (1891). "Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was performed for the relief of fluid pressure". Lancet 1 (3531): 981–2. doi:10.1016/S0140-6736(02)16784-5.
- ^ Quincke HI (1891). Verhandlungen des Congresses für Innere Medizin, Zehnter Congress, Wiesbaden. 10. pp. 321–331.
- ^ Quincke HI (1902). Die Technik der Lumbalpunktion. Berlin & Vienna.
- ^ Heinrich Irenaeus Quincke at Who Named It?
[edit] External links
| |||||||||||||||||||||||||||||||||
Retrieved from "http://en.wikipedia.org/wiki/Lumbar_puncture"
Subscribe to:
Posts (Atom)